propagation
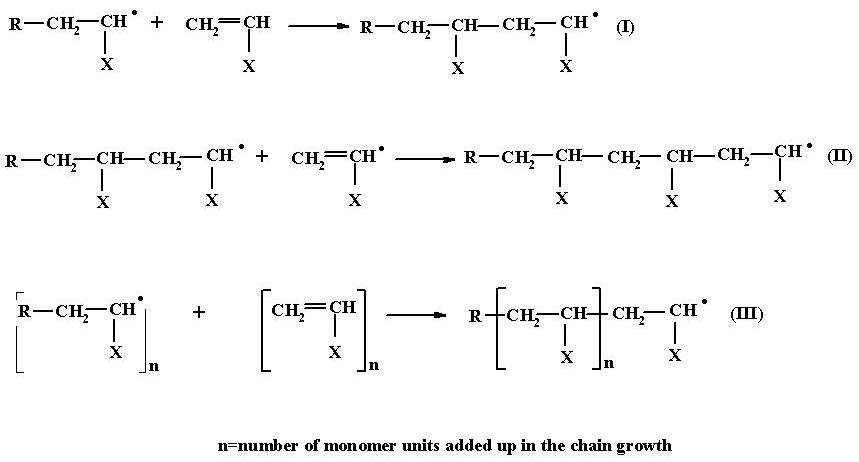
After initiation comes propagation. In the propagation step, the radical site at the first monomer unit attacks the double bond of a fresh monomer molecule. This results in the linking up of the second monomer unit to the first and the transfer of the radical site from the first monomer unit to the second, by the unpaired electron transfer process. (I)
This chain still contains a radical site at its end carbon atom and can therefore attack yet another monomer molecule with the simultaneous transfer o the radical site to the new monomer unit added. (II)
This process involving a continuous attack on the fresh monomer molecules which in turn, keep successively adding to the growing chain one after another is termed propagation. (III) The propagation lasts till the chain growth is stopped by the free radical site being killed by some impurities or by sheer termination process or till there is no further monomer left for the attack.
Each time a new monomer unit is added to the growing chain and there is an energy release of about 20 kcal. Hence, the process of chain propagation is brought about without adding any external energy to the system. In other words, a very small amount of energy is supplied to decompose the initiator so as to form its free-radical fragments and the polymer chain starts growing with large amounts of energy release.